Clinical trials conducted with involvement of the Copenhagen Trial Unit
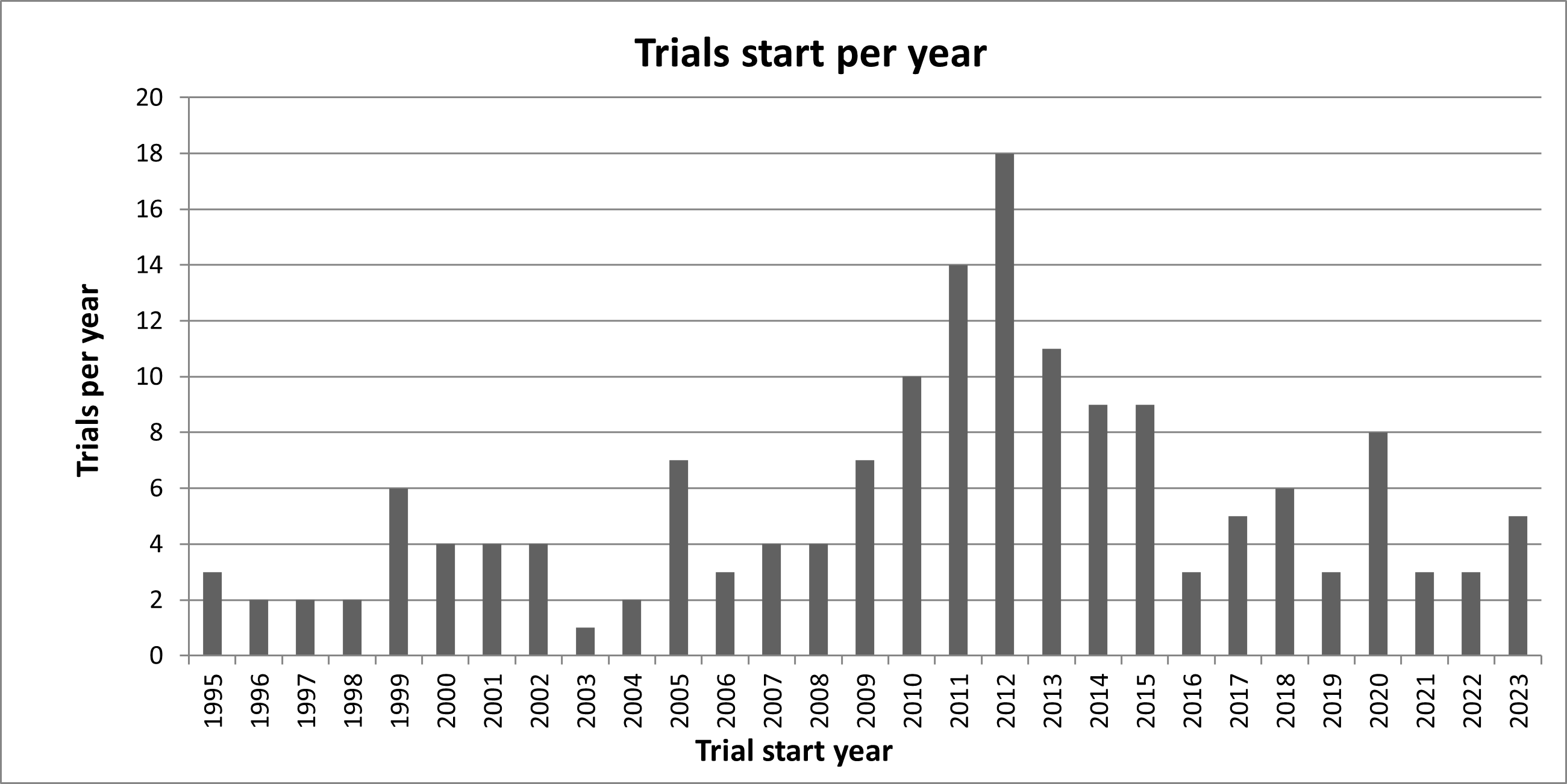
Number of randomised clinical trials launched each year
As a flexible partner and service provider, the Copenhagen Trial Unit becomes involved in new trials each year. The Copenhagen Trial Unit participates in the conduct of the trials with variable responsibilities, including all or some of the following: systeamtic review of the control interventions, systeamtic review of the experimental intervention, protocol development, regulatory affairs, ethical approval, randomisation, data management, statistical analyses, sponsor, and reporting. Since the establishment in 1995, the Copenhagen Trial Unit has been involved in the launch of more than 162 randomised clinical trials. The figure below shows the number of trials launched each year.
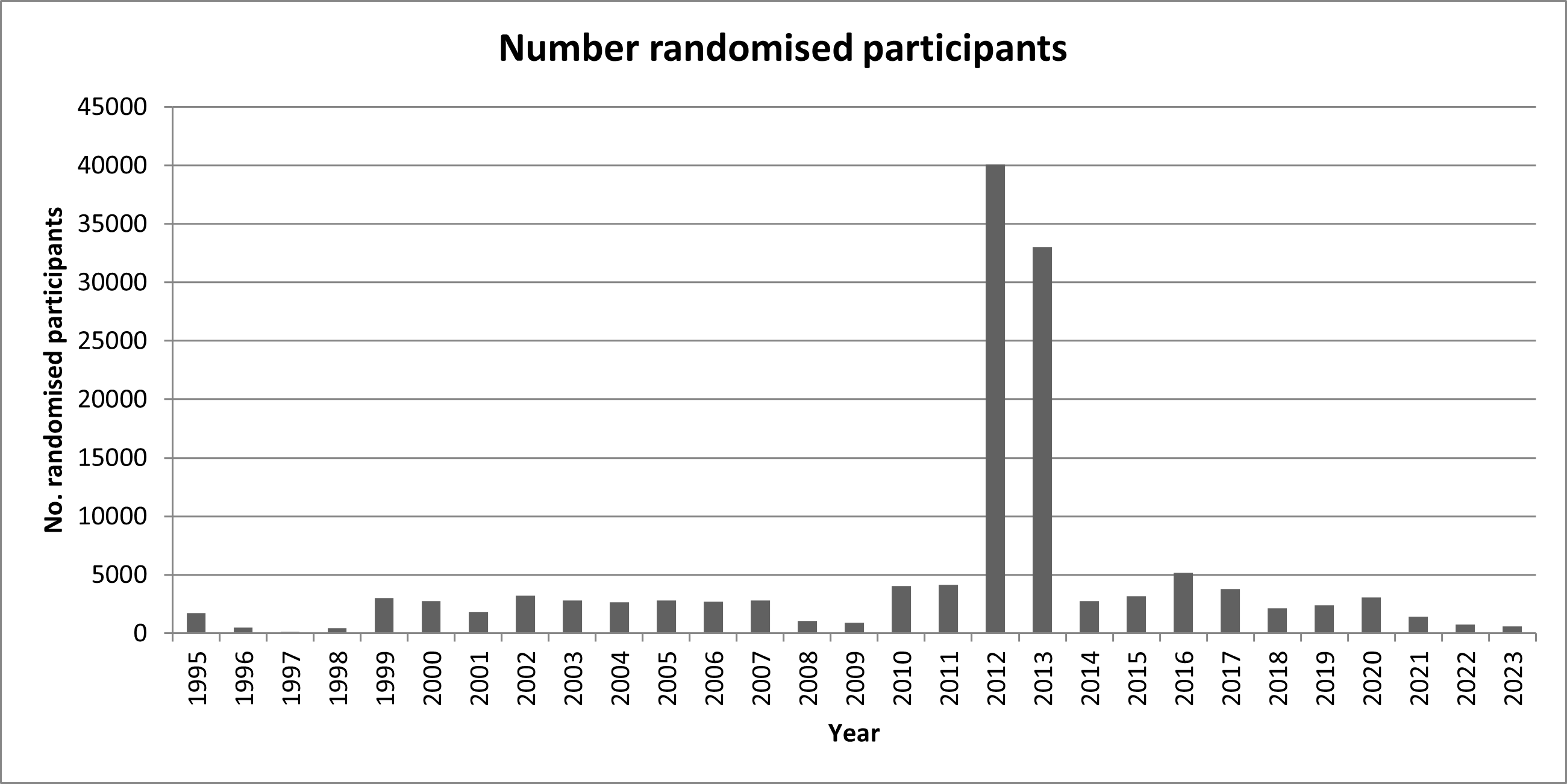
Number of participants randomised per year
In total, more than 135,000 participants have been included in the randomised clinical trials, as displayed below in the yearly graph. For more information, see the individual detailed trial pages.
GANTT overview of 162 randomised clinical trials
Download GANTT overview of the 162 randomised clinical trials launched in the years 1995-2024 here.
