History of the Copenhagen Trial Unit
Background for establishing the CTU
Denmark has a long tradition within health science research, achieving a strong position within the clinical research field (1-3).
Erik Juhl (born 22.4.1939 to 2.4.2024; https://da.wikipedia.org/wiki/Erik_Juhl) had a strong clinical and research background in hepatology (4,5,6). During the late 1980s he turned towards more senior organisational roles in the Danish health care system as director for the Copenhagen hospitals. He recognised the need for establishing a trial service unit within the hospital sector and started working for implementing such a unit in the early 1990s.
In 1995, the NASTRA-report 'Proposal for a national strategy for health science' was developed (7). The report recommended a strengthening of the Danish health science research by giving high priority to the areas where Danish research already had a strong position. One of the four areas recommended was clinical research with preventive, diagnostic, therapeutic, and nursing care objectives. The other three areas were genetic research, brain research, and preventive research.
Some of the currently applied interventions within the health care sector have not been subjected to adequate evaluation of the efficacy and socioeconomic impact. Many new interventions await to become assessed before being introduced into clinical practise. It is, therefore, important to support the research that evaluates the effect of the already implemented interventions and the research that evaluates the effect of new interventions before their implementation. The selection of appropriately designed studies – i.e., randomised clinical trials – becomes of central importance (8).
The European Good Clinical Practice (GCP) guidelines and EU directives and regulations for clinical research have contributed to the rise of clinical research to a higher standard but have also put a heavy workload on the clinical researchers. Collaboration between clinical researchers and clinical trial units, sharing the ambition to secure scientific quality in the conduct of trials, has become very important (8,9,10).
In order to evaluate the possibility of establishing a centre for clinical research within the Copenhagen Health Services, Christian Gluud visited more than 40 research centres in Europe and North America during 1993 (10). Extensive interviews among key researchers in these institutions were carried out. Based on these investigations, a report was prepared (10). The report was then circulated internationally and nationally for comments and eventually formed the basis for the proposal to establish the Copenhagen Trial Unit (CTU) (10).
Clinicians, various research institutions, and the drug industry, at home and abroad, supported the proposal for establishing a centre for clinical research. Many national organisations and institutions believed the establishment of a clinical trial unit could contributeto strengthen the Danish clinical research.
In October 1994, the Copenhagen City Council decided to establish the Copenhagen Trial Unit – the CTU. On June 1, 1995, Christian Gluud, was appointed Head of the Department. On January 1, 1996, after a phase of planning and preparation, the CTU was set up. During the first years, the CTU was core funded by the Copenhagen Health Services. Since 2007 the funding of the CTU has been taken over by the Danish state.
In 2019, the CTU and the Cochrane Hepato-Biliary Group (CHBG) underwent an international evaluation at the request of the Ministry for Health. The evaluation consisted of a self-evaluation questionnaire developed by VIVE (11), and an evaluation report based on site visits and interviews with employees, partners, and collaborators (12). The Evaluation Panel concluded, that the CTU had achieved its main goals: support, coordinate and conduct of randomised clinical trials; participation in the development of methods for randomised clinical trials and meta-analyses; education of students and researchers in evidence-based medicine, randomised clinical trials, meta-analyses and trial sequential analysis; and support, co-ordination, and conduct of systematic reviews of the literature. Furthermore, the CTU had published its research in a range of high impact medical journals. The impact was substantial as CTU-studies had influenced clinical practice and stimulated public debate and discussion. The productivity of both CTU and CHBG was high (12).
In June 2020, the Ministry of Health and Elderly informed that the governance of the CTU was to be changed, from Rigshospitalet to the Capital Region of Denmark, coming into effect January 1st, 2021 (13). The funding of the CTU was not changed. The CTU will remain at the usual working address.
References:
- Gluud C, Nikolova D. Likely country of origin in publications on randomised controlled trials and controlled clinical trials during the last 60 years. Trials. 2007: 8: 7. View.
- Wolff RF, Reinders S, Barth M, et al. Distribution of country of origin in studies used in Cochrane reviews. PLOS ONE. April 2011: 6(4): e18798. View.
- Herrmann KH, Wolff R, Scheibler F, et al. All nations depend on the global knowledge pool – Analysis of country of origin of studies used for health technology assessments in Germany. PLOS ONE. March 2013: 8(3): e59213. View.
- Sørensen TIAS, Madsbad S, Gluud C. Words of remembrance of Erik Juhl. Ugeskrift for læger 2024. https://ugeskriftet.dk/kolleganyt/erik-juhl-1 (in Danish)
- Henriksen JHS. Words of remembrance of Erik Juhl. Ugeskrift for læger 2024. https://ugeskriftet.dk/kolleganyt/erik-juhl (in Danish)
- Krogsgaard K. Words of remembrance of Erik Juhl. Ugeskrift for læger 2024. https://ugeskriftet.dk/kolleganyt/erik-juhl-0 (in Danish)
- The National Strategy Committee for Health Science Research (NASTRA). Proposal for a national strategy for health science. Report #1284. Copenhagen: Ministry of Research; 1995. View.
- Jakobsen JC, Gluud C. The necessity of randomized clinical trials. British Journal of Medicine & Medical Research. 2013;3(4):1453-68. View.
- Collins R, Bowman L, Landray M, et al. The magic of randomization versus the myth of real-world evidence; February 2020. View.
- Gluud C. Report on proposal to form a Clinical Trial Unit of the Copenhagen Health Services. Copenhagen: Copenhagen Health Services; November 1993. View.
- The staffs. Self-evaluation report of the Copenhagen Trial Unit, Centre for clinical Intervention Research, and the Cochrane Hepato-Biliary Group. 2019:1-145. View.
- Houlberg K, Hauge KM, Jensen MS, et al. Evaluering af Cochrane Denmark and Copenhagen Trial Unit. VIVE 2019: 66-91. View.
- Letter from the Permanent Secretary Per Okkels to the CTU (in Danish) June 2020. View.
Development per year of publications and citations as well as the Hirsch (h)-index
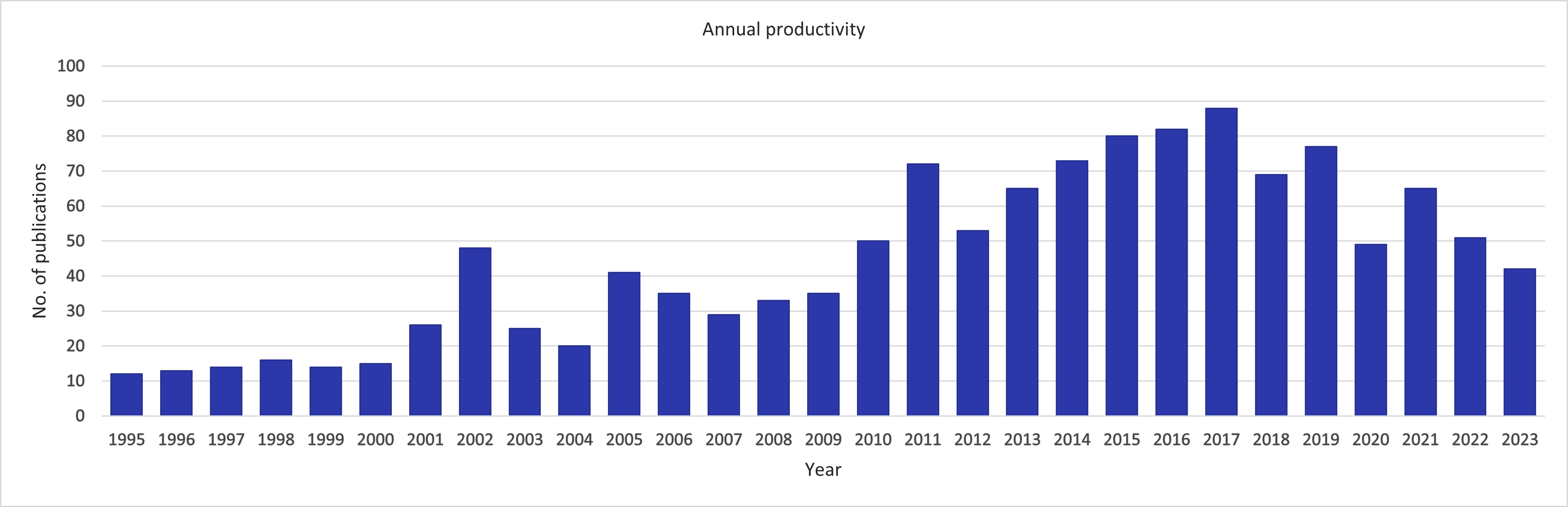
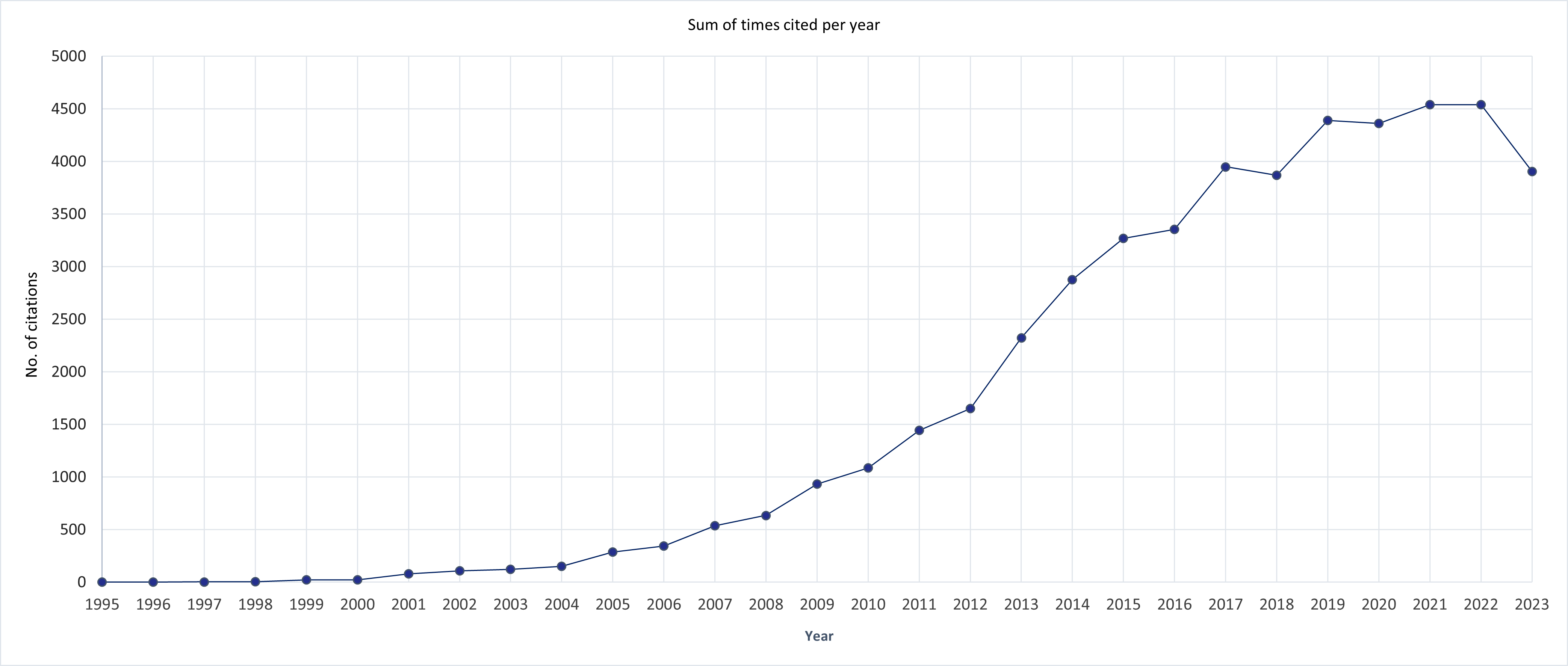
The h-index is 100 per 28th of December 2023.
